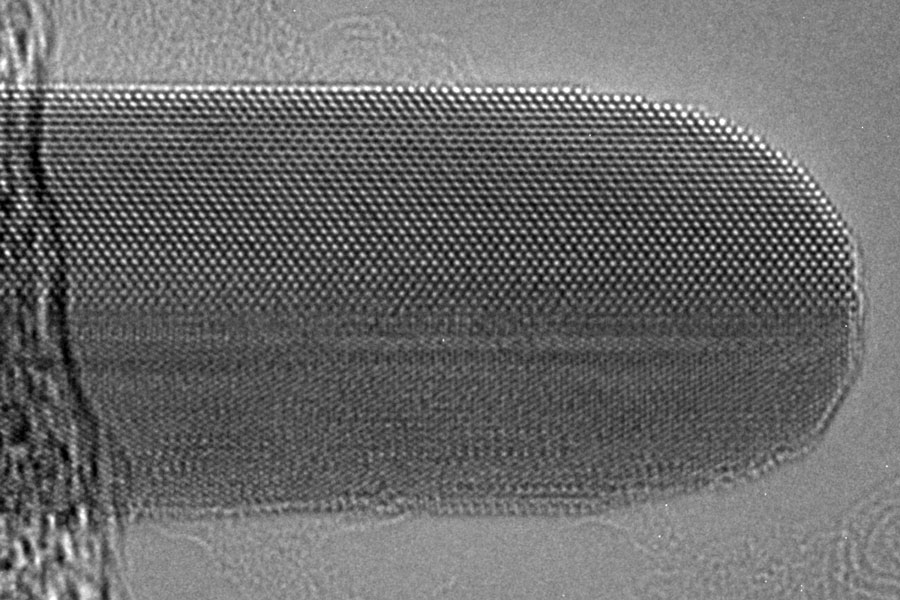
A research team from U of T Engineering has discovered previously unknown phenomena in the growth of nanoparticles. The insights could open new ways of engineering these tiny structures for a variety of purposes, from designing next-generation solar cells to developing new medical tests and treatments.
The team was led by recent graduate Paul Chen (ChemE PhD 2T2) and was a collaboration between the labs of Professor Frank Gu (ChemE, BME) and Professor Oleksandr Voznyy (Chemistry). In studying how atoms move to form rod-shaped gold nanoparticles, the researchers observed something that didn’t fit with the accepted theory.
“There are four modes of classical growth — that is, atom-by-atom growth — widely accepted in the literature,” says Chen, lead author of the new paper published in the Journal of the American Chemical Society reporting the team’s findings.
“But all of them assume the same thing, which is that atoms deposit onto a given facet — one side of the 3D shape — and generally end up integrating into that same facet. What we’ve shown, through multiple lines of evidence, is that this isn’t necessarily true.”
Using advanced methods that enabled them to capture images of how atoms move on these nanoparticles, the team showed that many atoms that originally deposited on the sides of the rod actually migrate to the ends of the rod, becoming part of the larger structure there instead.
Their experiments further uncovered exactly how the atoms migrated. Atoms moved toward the end of the rod in pairs before then hopping as individual atoms onto the end of the rod, where they integrate.
Such movement of atoms between nanoparticle facets has not been observed before, and the authors’ proposed mode of growth changes the established picture of how nanoparticles can form. It also offers a potential new paradigm for controlling the size and shape of nanoparticles.
Today, most nanoparticles in commercial use — such as the quantum dots that power high-definition video screens — are spherical. But according to Chen, other shapes offer intriguing possibilities.
“It’s possible to make rods, icosahedra, pyramids, nanoparticles that are shaped like popcorn, or even shapes called caltrops, which are spiky in all directions,” says Chen, who is now a Schmidt Science Fellow at the Massachusetts Institute of Technology and the Broad Institute of MIT and Harvard.
“All of these shapes have a higher surface-to-volume ratio than spheres and can exhibit new properties. When it comes to things like catalysis, a lot of important chemistry happens at the surface and with different shapes.”
Nanoparticle catalysts made of certain metals could help fight climate change by speeding up reactions that convert waste CO2 into fuels or other useful industrial chemicals. These catalysts could also offer pathways to making new materials, such as plastics that can biodegrade more easily, or that take less energy to produce than what is available today.
Nanoparticles are also used in medicine, either as part of diagnostic tests that rapidly check for the presence of diseases at the point of care, or as emerging therapies. For example, many researchers around the world are experimenting with gold nanorods that could be placed next to cancer tumours. These nanorods absorb laser light at certain key wavelengths, producing heat that kills the cancerous cells while leaving healthy ones alone.
Chen says that regardless of the application, understanding and controlling the shape of the nanoparticles as they grow will be key, but until now, physicists and chemists have had a limited understanding of these processes.
“Before this work, the main way to control nanoparticle shape was thought to be through passivation: basically, you coat some facets with ions or molecules that make it harder for atoms to deposit there, so they deposit on other facets instead. You influence where atoms deposit to direct growth,” says Chen.
“But what we’ve found is that you can also direct growth by influencing where atoms integrate, because deposition and integration can actually be de-coupled as two separate processes, as revealed in our work. Atoms that deposit on one facet can actually migrate to another facet to integrate. This potentially gives nanoengineers another knob to turn, so to speak, to make nanoparticles of the exact size and shape that they want.”
Chen says that even though the study investigated gold nanorods as a model system, similar phenomena could be found on other types of materials. Plausible candidates include other metals or semiconductors like perovskites, which are used in experimental solar cells.
“In the future, I think we’ll see more and more technologies developed through a deep, atomistic understanding of how these tiny structures form. By adding to that understanding, I hope that our work opens up new potential spaces for researchers to explore.”
This work was funded, in part, by the Natural Sciences and Engineering Research Council of Canada (NSERC). Other authors of the paper include Arianna Skirzynska (ChemE PhD candidate) and Tiange Yuan (Chemistry PhD candidate).