A new technology developed by researchers at the University of Toronto uses a microfluidic device combined with a novel airflow system to mimic the environment of lung airways. The device enables scientists and engineers to perform various particle exposure experiments to examine the pathological effects of air pollutants on respiratory health.
This development was recently published in the issue of Advanced Materials Technologies by BME PhD candidate Siwan Park and Professor Edmond Young (MIE, BME).
The microfluidic device-on-a-chip — called E-FLOAT, which stands for Extractable Floating Liquid gel-based Organ-on-a-chip for Airway Tissue modelling under airflow — is an easily modifiable system where scientists can grow lung cells in a suspended hydrogel that resembles lung tissue.
The researchers developed the device by micro-milling and bonding layers of thermoplastic. It incorporates a special channel geometry for growing the cells, and an airflow system connected to the device can generate various flow rates of warm and humidified air to simulate breathing in human lungs.
“We showed that lung airway tissue can be micro-engineered in the lab, exposed to various environmental conditions, including airflow and pollutants, and then be extracted for further interrogation as if it were a real lung tissue sample,” says Young.
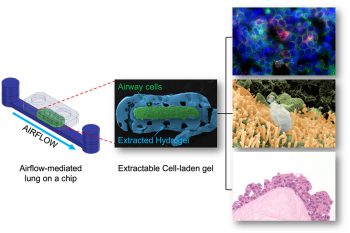
In many existing iterations of the technology, cells grown on microfluidic devices are limited to ‘on-chip’ analysis to assess the effect of external stimuli — such as airflow — on the health of the cells. This limits the analysis that can be carried out: while scientists can remove these cells from the device, this process changes the spatial location of the cells in relationship to the tissue, potentially skewing the results.
“One of the advantages of E-FLOAT is the ability to extract the biomimetic airway tissue that allows us to develop an in-depth knowledge through a wide array of imaging technologies,” says Park.
The researchers successfully delivered airborne particles onto the airway cells via controlled airflow to mimic how air pollutants would interact with lung cells. They then extracted the entire hydrogel and analyzed particulate and cell interactions.
“We were especially excited to obtain the stunning images of histology sections using the extracted hydrogel. Not only does it look beautiful, we believe that it may also be significant in histological and pathological perspectives. Also, depending on how we design the cell-matrix interactions in E-FLOAT, we may obtain a more physiologically accurate representation of multicellular airway tissue.”
“In the future, the plan is to use this technology to study the development of lung diseases like asthma — especially in the presence of air pollution — and to also use it as a preclinical model during drug development,” says Young.
“There is obviously a lot more work to be done, but we hope to collaborate with lung researchers and partner with pharma down the road to realize this plan.”




