Engineers at the University of Toronto just made assembling functional heart tissue as easy as fastening your shoes. The team has created a biocompatible scaffold that allows sheets of beating heart cells to snap together just like Velcro™.
“One of the main advantages is the ease of use,” says Professor Milica Radisic (ChemE, IBBME), who led the project. “We can build larger tissue structures immediately before they are needed, and disassemble them just as easily. I don’t know of any other technique that gives this ability.”
Growing heart muscle cells in the lab is nothing new. The problem is that too often, these cells don’t resemble those found in the body. Real heart cells grow in an environment replete with protein scaffolds and support cells that help shape them into long, lean beating machines. In contrast, lab-grown cells often lack these supports, and tend to be amorphous and weak. Radisic and her team focus on engineering artificial environments that more closely imitate what cells see in the body, resulting in tougher, more robust cells.
Two years ago, Radisic and her team invented the Biowire, in which heart cells grew around a silk suture, imitating the way real muscle fibres grow in the heart. “If you think of single fibre as a 1D structure, then the next step is to create a 2D structure and then assemble those into a 3D structure,” says Boyang Zhang a PhD candidate in Radisic’s lab. Zhang and Miles Montgomery, another PhD student in the lab, were co-lead authors on the current work, published today in Science Advances.
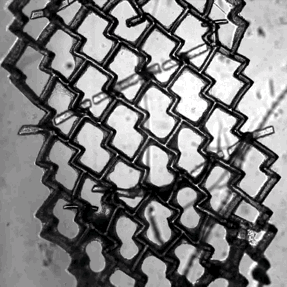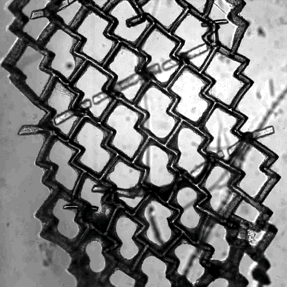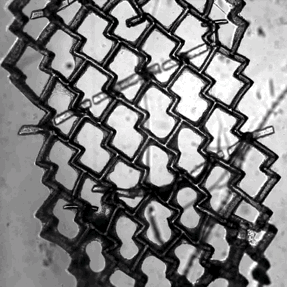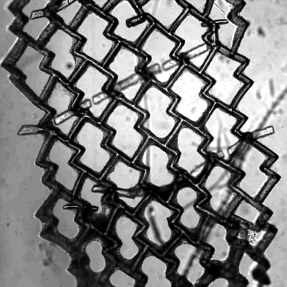
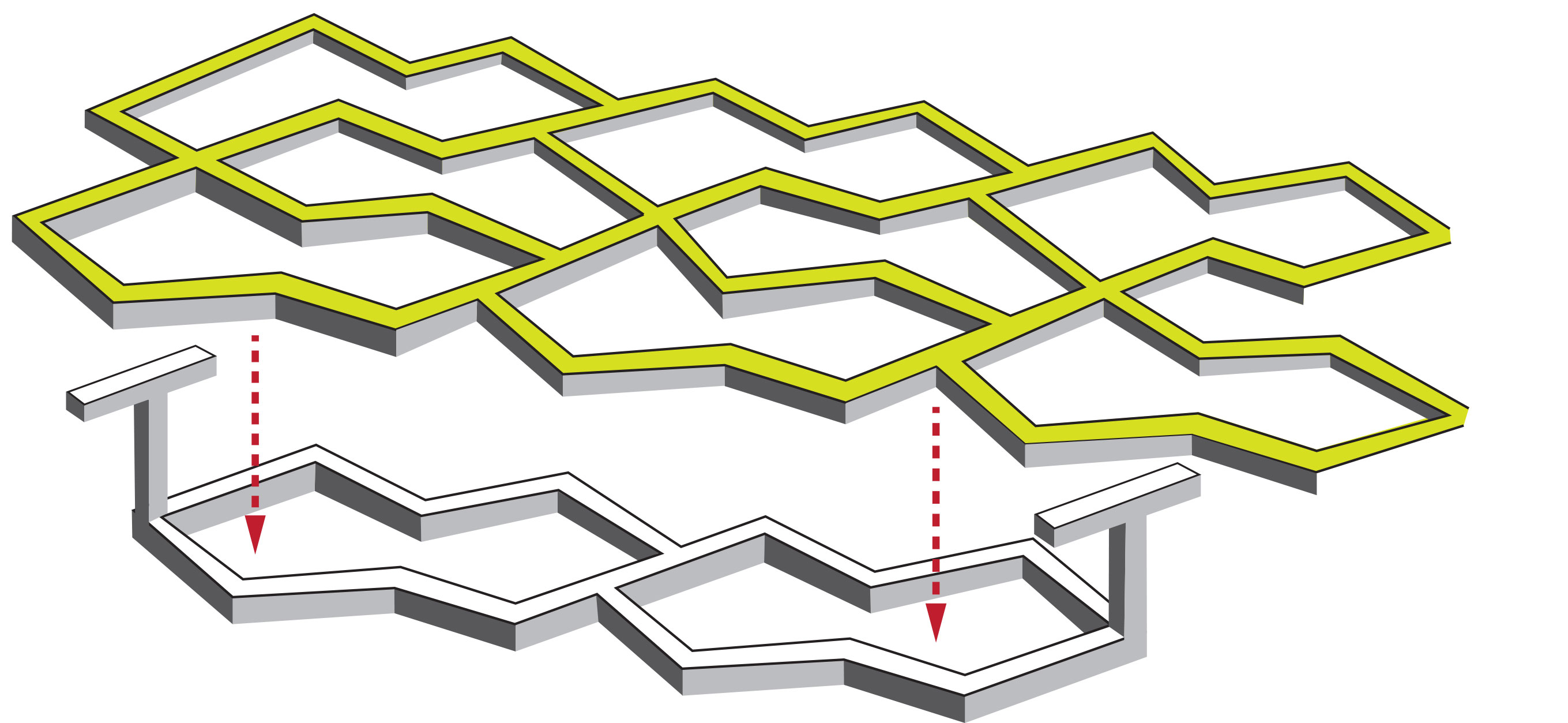
Zhang and his colleagues used a special polymer called POMaC to create a 2D mesh for the cells to grow around. It somewhat resembles a honeycomb in shape, except that the holes are not symmetrical, but rather wider in one direction than in another. Critically, this provides a template that causes the cells to line up together. When stimulated with an electrical current, the heart muscle cells contract together, causing the flexible polymer to bend.
Next the team bonded T-shaped posts on top of the honeycomb. When a second sheet is placed above, these posts act like tiny hooks, poking through the holes of honeycomb and clicking into place. The concept the same as the plastic hooks and loops of Velcro™, which itself is based on the burrs that plants use to hitch their seeds to passing animals.
Amazingly, the assembled sheets start to function almost immediately. “As soon as you click them together, they start beating, and when we apply electrical field stimulation, we see that they beat in synchrony,” says Radisic. The team has created layered tissues up to three sheets thick in a variety of configurations, including tiny checkerboards.
The ultimate goal of the project is to create artificial tissue that could be used to repair damaged hearts. The modular nature of the technology should make it easier to customize the graft to each patient. “If you had these little building blocks, you could build the tissue right at the surgery time to be whatever size that you require,” says Radisic. The polymer scaffold itself is biodegradable; within a few months it will gradually break down and be absorbed by the body.

Best of all, the technique is not limited to heart cells. “We use three different cell types in this paper; cardiomyocytes, fibroblasts and endothelial cells, but conceptually there is really no limitation,” says Radisic. That means that other researchers could use the scaffold to build layered structures that imitate a variety of tissues, from livers to lungs. These artificial tissues could be used to test out new drugs in a realistic environment.

Moreover, the ability to assemble and disassemble them at will could enable scientists to get much more detailed information on cell response than is currently possible. “You could take middle layer out, to see what the cells look like,” says Radisic. “Then you could apply a molecule that will cause differentiation or proliferation or whatever you want, to just that layer. Then you could put it back into the tissue, to see how it interacts with the remaining layers.”
The next step is to test how well the system functions in vivo. Radisic and her team are collaborating with medical researchers in order to design implantation experiments that will take the project one step closer to the clinic.