Researchers from U of T Engineering have discovered that an active, rather than passive, process dictates which nanoparticles enter solid tumours. The finding upends previous thinking in the field of cancer nanomedicine and points toward more effective nanotherapies.
The prevailing theory in cancer nanomedicine — an approach that enables more targeted therapies than standard chemotherapy — has been that nanoparticles mainly diffuse passively into tumours through tiny gaps between cells in the endothelium, which lines the inner wall of blood vessels that support tumour growth.
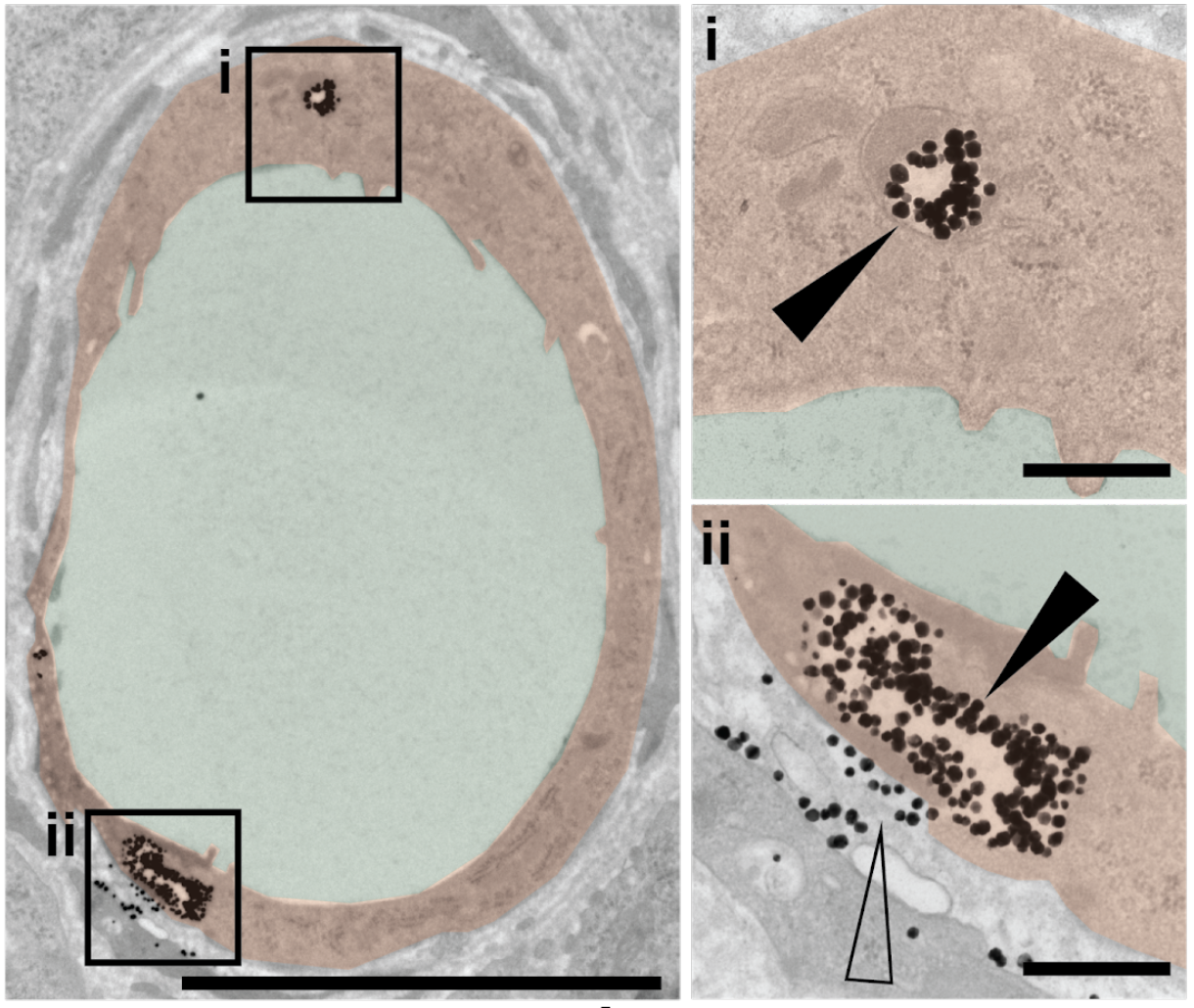
The researchers previously showed that less than one percent of nanoparticle-based drugs typically reach their tumour targets. In the current study, the team found that among nanoparticles that do penetrate tumours, more than 95 percent pass through endothelial cells — not between gaps among those cells.
“Our work challenges long-held dogma in the field and suggests a completely new theory,” says Abdullah Syed, a co-lead author on the study and postdoctoral fellow in the lab of Warren Chan, a professor at the Institute of Biomaterials and Biomedical Engineering (IBBME) and the Donnelly Centre for Cellular and Biomolecular Research.
The study was published today in Nature Materials.
“We saw many nanoparticles enter the endothelial cells from blood vessels and exit into the tumour in various conditions. Endothelial cells appear to be crucial gatekeepers in the nanoparticle transport process.”
Syed compares nanoparticles to people trying to get into popular restaurants on a busy night. “Some restaurants don’t require a reservation, while others have bouncers who check if patrons made reservations,” he says. “The bouncers are a lot more common than researchers thought, and most places only accept patrons with a reservation.”
The researchers established that passive diffusion was not the mechanism of entry with multiple lines of evidence. They took more than 400 images of tissue samples from animal models, and saw few endothelial gaps relative to nanoparticles. They observed the same trend using 3D fluorescent imaging and live-animal imaging.
Similarly, they found few gaps between endothelial cells in samples from human cancer patients.
The group then devised an animal model that completely stopped the transportation of nanoparticles through endothelial cells. This allowed them to isolate the contribution of passive transport via gaps between endothelial cells, which proved to be miniscule.
The researchers posit several active mechanisms by which endothelial cells might transport nanoparticles into tumours, including binding mechanisms, intra-endothelial channels and as-yet undiscovered processes, all of which they are investigating.
Meanwhile, the results have major implications for nanoparticle-based therapeutics.
“These findings will change the way we think about delivering drugs to tumours using nanoparticles,” says Shrey Sindhwani, also a co-lead author on the paper and an MD/PhD student in Chan’s lab. “A better understanding of the nanoparticle transport phenomenon will help researchers design more effective therapies.”
The research included collaborators from the Department of Physics (University of Toronto), Cold Spring Harbor Laboratory (New York, USA) and the University of Ottawa. The study was funded by the Canada Research Chairs Program, Canadian Cancer Society, Natural Sciences and Engineering Research Council, and Canadian Institutes of Health Research.



